Global neurovascular devices market is expected to reach USD 3.48 Billion by 2026, at a CAGR of 5.97% from 2019 to 2026. Growing preference for the minimally invasive surgeries, global increase in geriatric population and favorable medical reimbursement conditions are driving the global neurovascular devices market.
Market Overview:
Neurovascular devices are mainly used in the surgery, diagnosis and treatment of various neurological diseases. These devices are immensely used in the treatment and diagnosis of all categories of diseases and conditions involving the peripheral and central nervous system. Neurovascular devices aid in the treatment of various neurovascular disorders such as cerebral aneurysms and intracranial atherosclerotic disease. Thus, the rising incidence of the neurological disorders coupled with a growing demand for effective neurovascular devices are expected to drive the market growth over the forecast period.
Report Description:
Market Dynamics:
Drivers:
Restrains:
Opportunities:
Challenges:
Global Neurovascular Devices Market Key Findings:
All the segments have been analyzed on global, regional and country basis. The study includes the analysis of more than 30 countries for each segment.
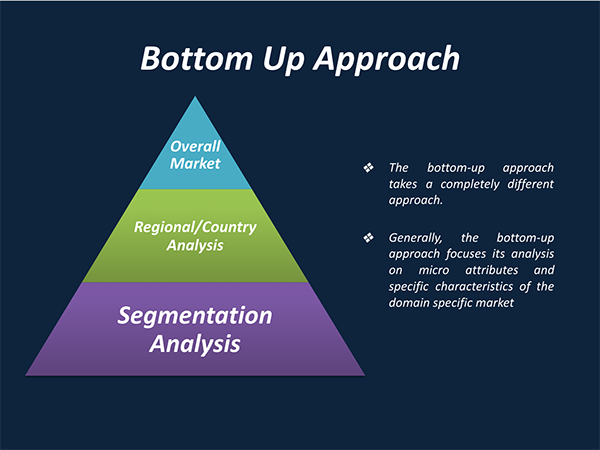
Segmentation Analysis:
The global Neurovascular Devices market has been segmented on the basis of device type, and therapeutic application.
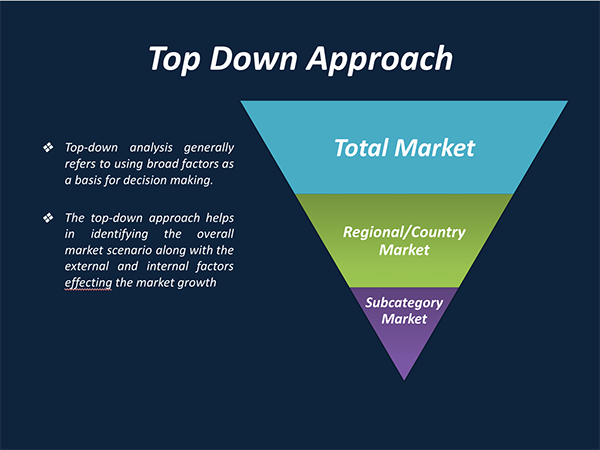
Regional Segmentation Analysis:
The regions analyzed for the market include North America, Europe, South America, Asia Pacific, and Middle East and Africa. The North America region emerged as the largest market for the neurovascular devices with a 42.18% share of market revenue in 2018.
Global Neurovascular Devices Market Competitive Analysis:
Key players in the global neurovascular devices market are Abbott Laboratories, W. L. Gore & Associates, Inc., Johnson & Johnson, Medtronic PLC, Merit Medical Systems, Inc., MicroPort Scientific Corporation, Penumbra, Inc., Stryker Corp., Terumo Medical Corporation, Integer Holdings Corporation, and Acandis GmbH & Co. KG among others. Major players are focusing on new product launch in order to effectively participate in the highly competitive market. For instance in 2016, Penumbra, Inc. launched ACE 68 Reperfusion Catheter for the treatment of acute ischemic stroke. It includes latest technological advancements to deliver maximum aspiration power for extracting thrombus in acute ischemic stroke patients.
*All our reports are customizable as per customer requirements
This study forecasts revenue and volume growth at global, regional, and country levels from 2016 to 2026. Fior Market Research has segmented the global neurovascular devices market on the basis of below mentioned segments:
Global Neurovascular Devices Market by Device Type:
Global Neurovascular Devices Market by Therapeutic Application:
Global Neurovascular Devices Market by Region:
LIST OF TABLE
LIST OF FIGURES
Market research is a method of gathering, assessing and deducing data & information about a particular market. Market research is very crucial in these days. The techniques analyze about how a product/service can be offered to the market to its end-customers, observe the impact of that product/service based on the past customer experiences, and cater their needs and demands. Owing to the successful business ventures, accurate, relevant and thorough information is the base for all the organizations because market research report/study offers specific market related data & information about the industry growth prospects, perspective of the existing customers, and the overall market scenario prevailed in past, ongoing present and developing future. It allows the stakeholders and investors to determine the probability of a business before committing substantial resources to the venture. Market research helps in solving the marketing issues challenges that a business will most likely face.
Market research is valuable because of the following reasons:
Our research report features both the aspects; qualitative and quantitative. Qualitative part provides insights about the market driving forces, potential opportunities, customer’s demands and requirement which in turn help the companies to come up with new strategies in order to survive in the long run competition. The quantitative segment offers the most credible information related to the industry. Based on the data gathering, we use to derive the market size and estimate their future growth prospects on the basis of global, region and country.
Our market research process involves with the four specific stages.
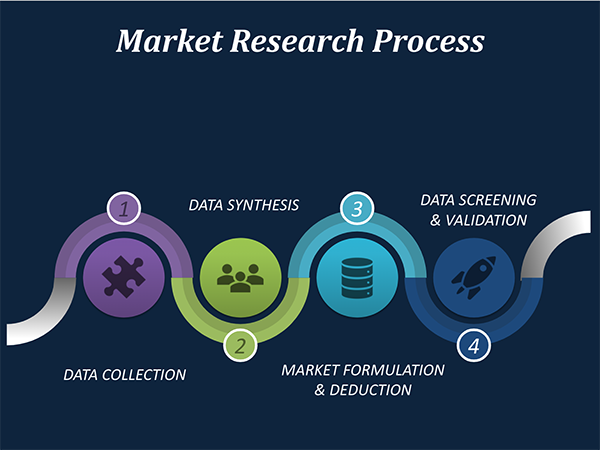
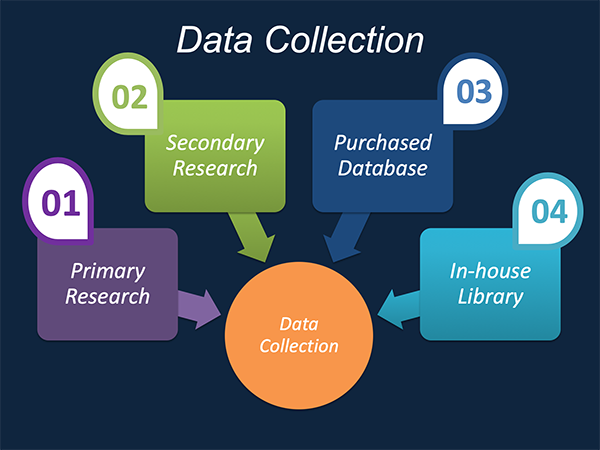
Data Collection: This stage of the market research process involves with the gathering and collecting of the market/industry related data from the sources. There are basically two types of research methods:
Data Synthesis: This stage includes the evaluation and assessment of all the data acquired from the primary and secondary research. It likewise includes in evaluating the information for any disparity watched while information gathering identified with the market. The data & information is gathered with consideration to the heterogeneity of sources. Scientific and statistical methods are implemented for synthesizing dissimilar information sets and provide the relevant data which is fundamental for formulating strategies. Our organization has broad involvement with information amalgamation where the information goes through different stages:
Market Formulation & Deduction: The last stage includes assigning the data & information in a suitable way in order to derive market size. Analyst reviews and domain based opinions based on holistic approach of market estimation combined with industry investigation additionally features a crucial role in this stage.
This stage includes with the finalization of the market size and numbers that we have gathered from primary and secondary research. With the data & information addition, we ensure that there is no gap in the market information. Market trend analysis is finished by our analysts by utilizing data extrapolation procedures, which give the most ideal figures to the market.
Data Validation: Validation is the most crucial step in the process. Validation & re-validation through scientifically designed technique and process that helps us finalize data-points to be used for final calculations. This stage also involves with the data triangulation process. Data triangulation generally implicates the cross validation and matching the data which has been collected from primary and secondary research methods.













































Free Customization
Countries can be added on demand
Free yearly update on purchase of Multi/Corporate User License
Companies served till date

We serve our customers 24x7 for 365 days through calls, emails and live chat options.
Huge database of exceptional market reports bringing market intelligence to your fingertips.

SSL enabled, we offer you various secured payment options for risk free purchase.